Interdisciplinary research for
an integrated
community-directed
strategy to eliminate malaria in tropical Africa
Interdisciplinary Research for an Integrated Community-directed Strategy to Eliminate Malaria in Tropical Africa
Project Overview
Research Background
In order to eliminate malaria, which remains an urgent issue for humanity, this research project aims to elucidate the transmission mechanisms on the individual and population levels and to conduct field studies of medical and economic interventions through multidisciplinary research, and to develop an integrated community-driven malaria elimination strategy tailored to local characteristics.
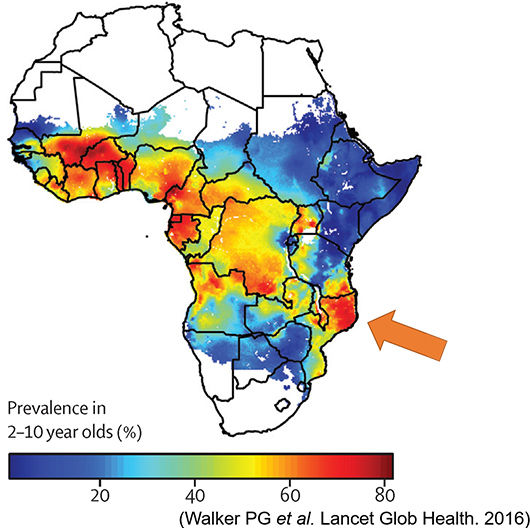
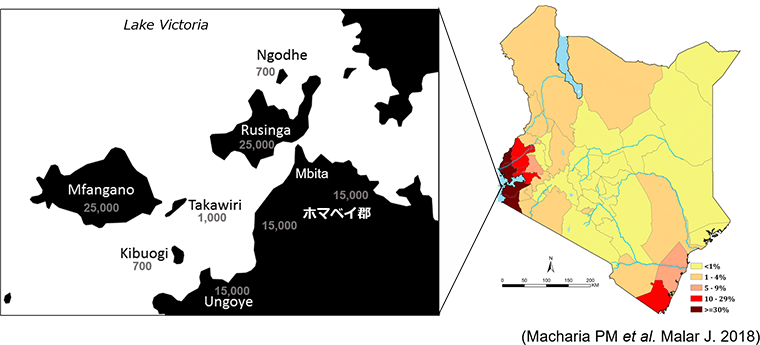
We have continuously conducted malaria surveys since 2012 in the area around Lake Victoria, Kenya, which is located at the eastern end of Africa's highly endemic "malaria belt", and found three problems behind the persistent transmission.
- Asymptomatic infection: In endemic areas, there are many people, mainly adults, who have acquired immunity through frequent malaria infections and are not showing any symptoms even though they are infected. These infected people are not tested for malaria because they do not have symptoms, and even if they are tested, they are not properly diagnosed because of the low level of parasite infection. Therefore, without being aware of it, these asymptomatic infected people become the source of infection in children with immature immunity, which can result in severe malaria.
- Residents' behavioral issues: As indicated by the inappropriate use of mosquito nets, inappropriate behaviors towards malaria prevention among local residents have long been considered a major issue in malaria control. We believe that this is due not only to a lack of appropriate knowledge about infection prevention and early diagnosis and treatment, but is also related to the cost of long-term malaria infection and the cost of short-term malaria prevention and treatment-seeking behavior.
- Insecticide-resistant vector mosquitoes: At the same time that malaria parasites have acquired resistance to antimalarial drugs, Anopheles vector mosquitoes have also been reported to have acquired resistance to insecticides, mainly those used in insecticide-treated nets.
Research Contents
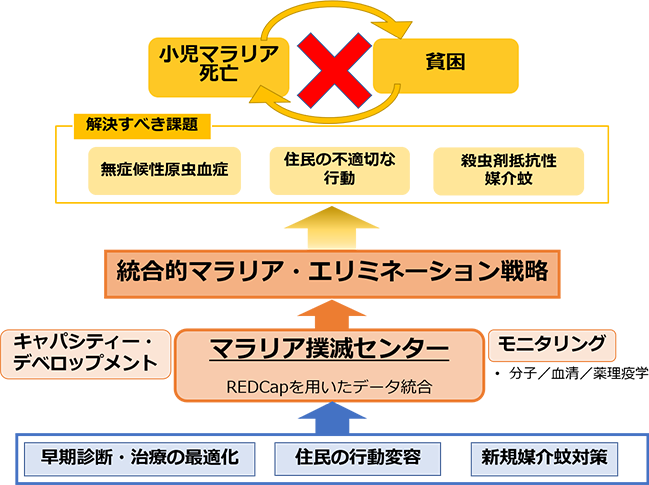
Based on the issues identified from the field observations and surveys, we are considering the following four policies for our activities.
- Optimization of early diagnosis and treatment: We will generate knowledge that will lead to early diagnosis and treatment of the population through the development of new diagnostic methods for malaria, analyses of genetic polymorphisms related to treatment outcome and disease severity among residents of the target area, and further strengthening of the local public health system.
- Behavioral change of residents: In addition to raising awareness of the knowledge and cost of malaria prevention and treatment, we will introduce a system to encourage proper malaria prevention practices among residents using the theory of behavioral economics, and evaluate its effectiveness through field trials.
- New vector control measures: In collaboration with Sumitomo Chemical and Nagasaki University, we will assess the effectiveness of ceiling nets that cover the openings between the roof and walls of local houses with mosquito net materials. Furthermore, by using a mosquito net material that is also effective against insecticide-resistant vectors, we expect to further suppress malaria transmission.
- Monitoring: We will monitor malaria transmission and incidence from multiple perspectives, including molecular epidemiology, genetics, serology, medical entomology, and socioeconomics, while simultaneously describing the true nature of asymptomatic transmission and heterogeneity in transmission intensity, and evaluating the new interventions described above.
We will set up a Center for Malaria Elimination in the target area to promote multinational researcher exchanges and skills transfer and develop sustainable control strategies led by local researchers and communities.

Expected Achievements and Significance of the Research
- We will establish a new community-driven malaria control strategy through interdisciplinary research, and aim to reduce the malaria burden in the target areas by deploying this strategy. The results are expected to be widely applied to the "malaria belt" of tropical Africa and contribute to the elimination of the vicious cycle of poverty and malaria.
- It is expected that knowledge on malaria transmission and mechanisms of pathogenesis on the individual and population levels will lead to further development of countermeasures for malaria elimination. We will also promote capacity development of local researchers through researcher exchanges.
【At Mount Kenya University with Prof. SW Waudo, Vice Chancellor (center)】
Malaria Elimination
in the Pacific Islands
Malaria Elimination in the Pacific Islands: Development of an Integrated Community-directed Elimination Strategy against Plasmodium vivax in Vanuatu
Project Overview
The global eradication of malaria has once again become a priority. In this context, the greatest barriers are the large number of under-five children dying from Plasmodium falciparum in sub-Saharan Africa and the elimination of P. vivax outside that region. The latter includes Vanuatu, a group of 68 islands in the southwest Pacific Ocean. After infection by a vector mosquito, P. vivax remains in the liver as a dormant body for a long period of time, causing recurrent infections. There is no way to detect this dormant stage. Also, existing treatments are not effective enough to eliminate this parasite. Our intervention study in Vanuatu, which has been ongoing since 1987, revealed the following obstacles faced by the local malaria elimination program: low sensitivity of existing diagnostic methods, insufficient public awareness and community participation, and the need to optimize antimalarial treatment. Vanuatu has set a national goal of sustainable malaria elimination throughout the archipelago by 2025. This study supports the achievement of that goal and proposes the following activities:
- Apply behavioral economics theory to encourage appropriate malaria prevention and early treatment behaviors among residents, and create a community-driven mechanism to achieve malaria elimination.
- Support and scale up a PCR-based malaria surveillance system that detects even asymptomatic low-density infections, and integrate PCR-based diagnostic results into the existing District Health Information System to strengthen malaria case surveillance.
- To clarify the relationship between CYP2D6 polymorphisms, its enzymatic activity, and therapeutic efficacy and side effects of primaquine in order to promote the widespread use of primaquine as a curative treatment for P. vivax.
This interdisciplinary project, which incorporates social sciences and medicine in an interdisciplinary approach, will create a new integrated national strategy for malaria elimination in Vanuatu. In addition, the results of the project will serve as a model for wider implementation of malaria elimination in other island countries in the Pacific and Southeast Asia.
Malaria in Vanuatu
At the southeastern tip of Vanuatu is the Buxton Line, which forms the boundary of the range of Anopheles vector mosquitoes and malaria transmission in the Pacific (Figure 1). Malaria transmission in the region is thought to have continued since humans and malaria parasites emerged in Africa about 200,000 years ago and reached and settled in Vanuatu by about 3,500 years ago [Kaneko et al. 1998]. Historically, both P. falciparum and P. vivax were prevalent on all islands except Futuna, where there were no vector mosquitoes. P. falciparum incidence fluctuated seasonally with rainfall, reaching a peak between February and April. P. vivax incidence was more uniform throughout the year and was thought to reflect relapses from liver hypnozoite. Malaria prevalence is higher in the northern islands than in the southern islands [Kaneko et al. 1998].
In 1991, the possibility of malaria elinimation through an integrated package was tested on Aneityum, the southernmost island of Vanuatu. Subsequent studies to date have shown that malaria this isolated island can be eliminated with short-term mass drug administration, sustained vector control, and high levels of community awareness and participation (Kaneko et al. 2000). As a result of the scale-up of malaria control tools such as insecticide-treated mosquito nets and improved diagnostic and therapeutic drugs throughout Vanuatu since the beginning of this century, malaria has been remarkably controlled. The Annual Parasite Incidence (API), defined as the number of confirmed malaria cases per 1,000 people per year, was above 100 on many islands before 2000, but declined to 36.5 in 2010 and 2.2 in 2018. With the decline in API, the proportion of cases with P. vivax shifted to a predominance of 74% in 2018 [WHO 2019].
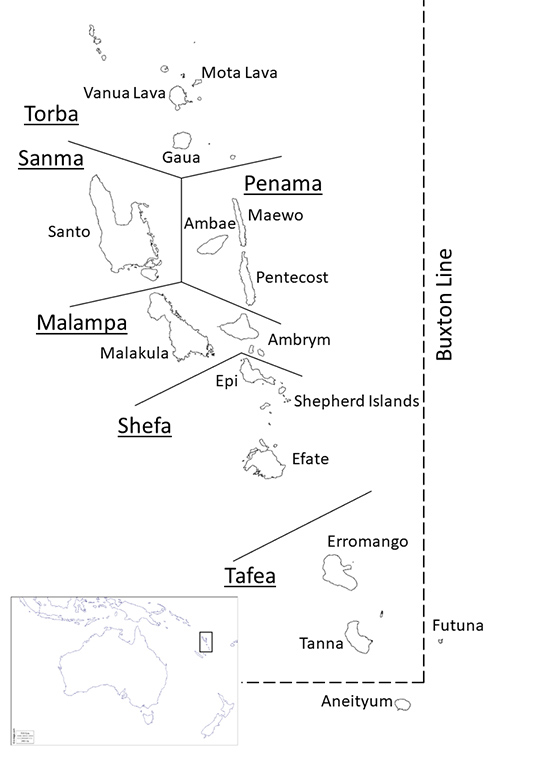
Vanuatu has set a national goal of achieving malaria elimination in the entire archipelago by 2025. The approach is to gradually expand malaria control from Tafea and Torba Provinces in the south and north, respectively, to the central and northern islands (Figure 1). In 2017, Tafea was declared malaria-free, having achieved zero cases of indigenous malaria in the previous three years, and Torba is close to achieving the same. Currently, most of the reported malaria cases are concentrated in the two provinces of Sanma and Malampa [WHO 2019].
Since 2002, we have conducted cross-sectional malariometric surveys in 38 villages on 12 islands in Vanuatu. Of a total of over 27,000 tests, 346 (1.3%) were positive by microscopy and 1656 (6.2%) were positive by PCR. Particularly on Ambae Island of Panama Province (Figure 1), six surveys were conducted between 2002 and 2014 to estimate malaria infection rates. Malaria infections were examined by microscopy at the survey sites, followed by PCR in the laboratory using dried blood spots on filter paper. Both microscopy and PCR showed an overall reduction in infection rates, consistent with the downward trend in malaria case rates observed in the health facilities described above (Figure 2). A comparison of detection methods showed that as the overall infection rate decreased, the proportion of sub-microscopic infections (PCR positive but negative microscopy) increased from 70.1% in 2002 to 100% in 2011 and 2014. Most of these low-density infections were asymptomatic.
The most recent surveys of infection rates on 11 islands across six provinces in Vanuatu since 2014 have confirmed the prevalence of low-density P. vivax infection that is undetectable by microscopic examination, highlighting the importance of employing molecular diagnostics to accurately identify residual infection hotspots. In this study, no malaria infection was detected by PCR in the southern Tafea Province, confirming that malaria eradication has been sustained. Furthermore, the infection rates of P. vivax in the northern provinces are not uniform, with higher rates found on Maewo Island (2015), Santo Island (2017), and Malakula Island (2017 and 2018).
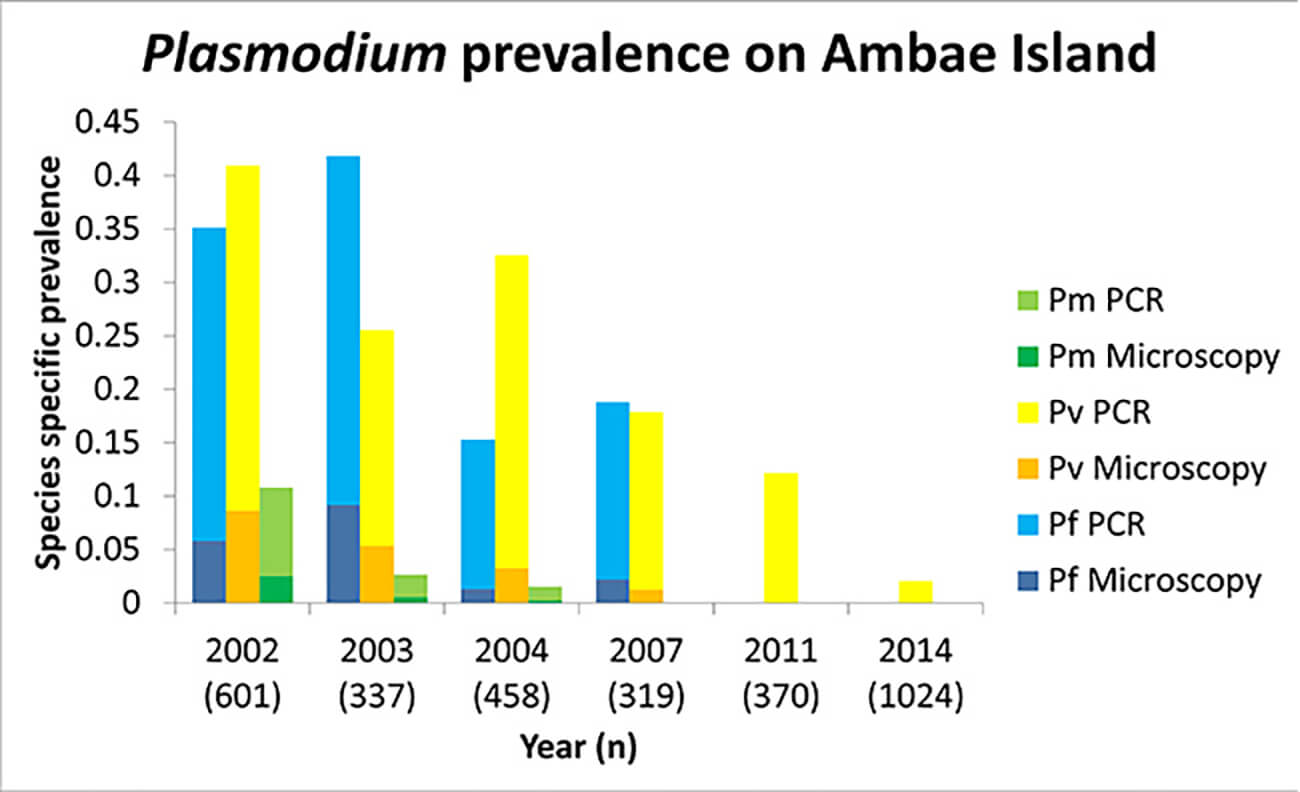 Figure 2: The trends of species-specific malaria prevalence in Ambae.
Figure 2: The trends of species-specific malaria prevalence in Ambae.Comparison based on diagnostic methods showed that, as the overall prevalence declined, the percentage of "low-density (sub-microscopic)" infections increased; from 70.1% in 2002 to 100% in 2011 and 2014. To add, the majority of these "low-density (sub-microscopic)" infections were completely asymptomatic.
From the survey results since 2014, low-density P. vivax infection has prevailed in Vanuatu, indicating the importance of applying molecular diagnostic methods in the area in order to identify the "hotspots" of residual transmission. In the islands of Tafea, the southern part of Vanuatu, no malaria was detected by PCR-based molecular methods and elimination had been maintained. On the other hand, The northern islands showed variable P. vivax infection rates, among which the three islands of Maewo, Santo, and Malakula showed the highest.
Challenges to Overcome
As the incidence of malaria declines in Vanuatu, the detection and elimination of remaining hotspots and the prevention of malaria reintroduction from outside are urgent issues, requiring effective surveillance and rapid response to confirmed cases. It is recommended that the annual blood examination rate (ABER), defined as the number of malaria tests performed per 100 people at risk of infection per year, be maintained at a minimum of 10%, but achieving this will require a high level of awareness among the population in the context of declining incidence.
Rapid diagnostic tests (RDTs) are the main diagnostic method for malaria in Vanuatu today. RDT detects the presence of malaria parasite antigens by antigen-antibody reaction on nitrocellulose strips, but a more sensitive diagnostic method is essential for detecting low-density P. vivax infection. We have started to introduce PCR and secure necessary human resources at Port Vila Central Hospital in collaboration with the local Ministry of Health since 2019 (photo).
Primaquine has been approved for use in Vanuatu since 2007 as an anti-hypnozoite agent for P vivax. Its use in health care settings has been slowed by concerns about G6PD deficiency, which carries the risk of inducing intravascular hemolysis upon primaquine intake as a side effect, although this is rare. In addition, the long 14-day treatment period means that few people complete primaquine treatment except under direct observation, resulting in persistent P. vivax infection and an increased risk of relapse.
The activity of CYP2D6, an isozyme of hepatic cytochrome P450, is important for primaquine metabolism. Although recent studies have examined the relationship between genotypic and phenotypic diversity of CYP2D6 and primaquine treatment [Baird et al. 2018; Brasil et al. 2018; Chen et al. 2019; Pett et al. 2019; Spring et al. 2019] the genetic diversity of CYP2D6 in Pacific Island populations and its impact on the efficacy and side effects of primaquine as an anti-hypnozoite agent is not known [Gutiérrez Rico et al. 2020].

Primaquine has been approved for use in Vanuatu since 2007 as an anti-dormant agent for P. vivax. Its use in health care settings has been slowed by concerns about G6PD deficiency, which has the risk of inducing intravascular hemolysis as a side effect, although this is rare. In addition, the long 14-day dosing period means that few people complete primaquine treatment except under direct observation, resulting in persistent P. vivax infection and an increased risk of relapse.
The enzymatic activity of CYP2D6, an isozyme of liver cytochrome P450, is essential in Primaquine metabolism. The relationships between genetic polymorphisms in the CYP2D6 gene and responses or phenotypic expression against primaquine treatment have been studied [Baird et al. 2018; Brasil et al. 2018; Chen et al. 2019; Pett et al. 2019; Spring et al. 2019]. However, the CYP2D6 polymorphisms within the people originating from the Pacific Islands and their effects on efficiency and adverse reactions of primaquine therapy is yet to be elucidated [Gutiérrez Rico et al. 2020].
Current malaria control programs assume that if a control tool is provided free of charge, people will make the best use of it. However, this assumption breaks down, especially when the malaria incidence rate declines, as it has in Vanuatu. The cost perception of malaria may be changing, along with the motivation to use mosquito nets (see photo) and to seek early diagnosis and treatment. The complex interplay of medical and behavioral economic factors such as asymptomatic transmission, acquired immunity, low knowledge, positive externalities of individual behavior, and extreme poverty need to be considered. To address this inappropriate behavior of the population, this study will utilize the theory of "behavioral economics" to examine mechanisms to promote behavioral change in the population, such as the introduction of incentive schemes for increasing awareness, preventive measures, and early treatment (specifically, a reward system for not contracting malaria). The challenge is to eliminate malaria through an integrated strategy in the target areas.

Significance of the Research
Vanuatu is a small island nation in the southwest Pacific, but achieving nationwide malaria elimination in the next five years could be a landmark achievement for island nations that share the problem of P. vivax elimination in Southeast Asia and Melanesia, including the Philippines, Indonesia, Papua New Guinea (PNG), and the Solomon Islands. New Guinea (PNG, Papua and West Papua Provinces of Indonesia) is the only highly malaria endemic region outside Africa.
Incorporating more sensitive detection methods, such as PCR, into routine case surveillance will allow more accurate assessment of progress in malaria elimination, including among asymptomatic infected individuals. Optimization of primaquine use will accelerate P. vivax elimination by reducing the number of hypnozoite carriers. In addition, this interdisciplinary research will develop an innovative integrated system that focuses specifically on human behavior through a local approach that combines medicine and behavioral economics. We believe that the results of this research will be implemented globally as an innovative P. vivax elimination strategy that is not limited to Vanuatu.
Translational Science for
Emerging & Re-emerging Infectious Disease Control
R&D towards Chagas
Disease Control
R&D towards Chagas Disease Control
Project Overview
Chagas disease, prevalent in Central and South America, is triggered by an infection caused by the parasite, Trypanosoma cruzi. The symptomatology of Chagas disease is unique in that regional differences exist in its manifestation. In Central America, there are many areas where heart diseases such as chronic myocarditis, dilated cardiomyopathy, and lethal arrhythmia occur frequently, but in South America, digestive diseases such as megaesophagus and megacolon are the main diseases. In many cases, organ damage is revealed after a latent phase, which can be as long as 30 to 40 years. The pathogenesis of Chagas disease and the mechanism of organ tropism of the trypanosome have not been clarified.
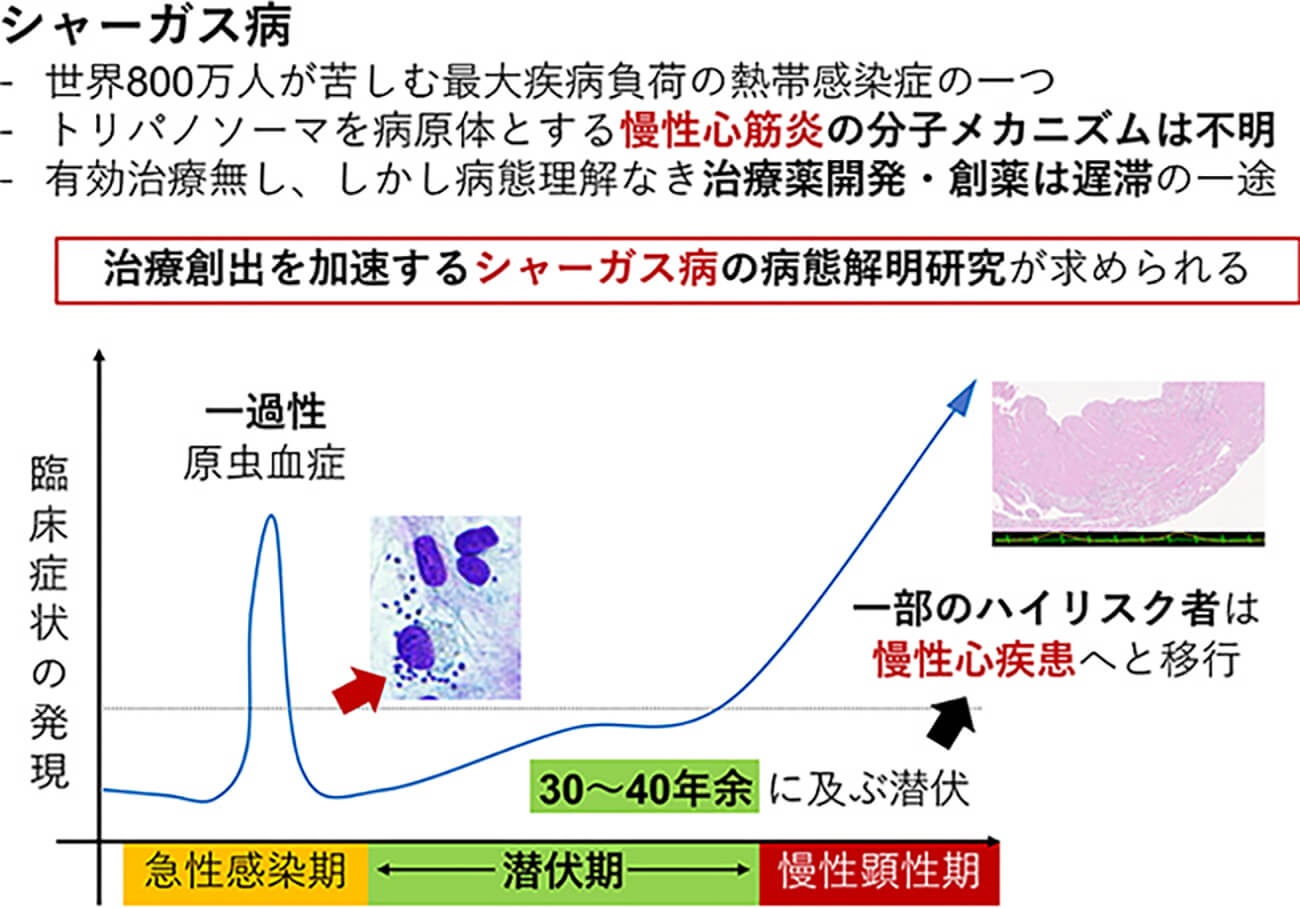
Who progresses to exhibit a prominent heart disease? What are the risk factors? These are both yet to be answered questions. The present therapeutics are not only of limited efficacy but also harbor high adverse effect rates. Novel therapeutics are of urgent need.
In the Project, with the support and collaboration of the National Center for Scientific Research of El Salvador (CICES), we will
- investigate the virulence factors of the trypanosome by a molecular genetics approach
- model Chagas cardiomyopathy, in vitro and in vivo
- synthesize and develop novel therapeutic agents against Chagas disease
The Project has been supported by the Japanese Science and Technology Research Partnership for Sustainable Development (SATREPS) program, since 2017.

Expected Achievements
Unveiling the virulence factors contributing to Chagas disease pathogenesis will boost the research and early clinical development of Chagas disease countermeasures. The Project will nourish human resource developmentin both countries and contribute to Chagas disease control in endemic countries.

Collaborating Institutions
El Salvador: CICES, University of El Salvador, University of Dr. José Matías Delgado, National Rosales Hospital
Japan: Gunma University, Tokyo University, Keio University, Takasaki University of Health and Welfare, Jichi University
リンク
Research Consortium
on COVID-19
Research Consortium on COVID-19
Project Overview
The world is facing a public health emergency due to the COVID-19 pandemic. The pandemic has threatened not only the affected individuals and their families, but also a wide variety of socioeconomic and cultural activities throughout the globe. We aimed to tackle the COVID-19 pathophysiology by establishing a reliable serological diagnostic method and unveiling the kinetics of the host humoral immune response.
Understanding the characteristics and kinetics of antibodies which the human host will produce in response to a viral invasion, is critical in establishing definitive diagnosis and effective treatment strategies against the virus. The emergence of antibodies targeting the epitopes responsible in viral neutralization, not only predicts viral clearance from an affected individual, but also indicates possibilities for becoming therapeutic plasma donor candidates. Establishing a reliable method for neutralizing antibody titeration and clarifying the relationships of serokinetics and clinical evolution shall contribute in efficient diagnostics, treatment stratification and transmission prevention.
We developed an immunofluorescence-based, quantitative, rapid, point-of-care, serological diagnostic for SARS-CoV-2 in collaboration with Mokobio Biotechnology R&D Center Inc (USA). The amount of blood needed for testing is as small as 20 μL. By its unique fluorescence-based semi-quantitation, the novel method avoids inter-observer judgment errors by digitizing the measurement process.
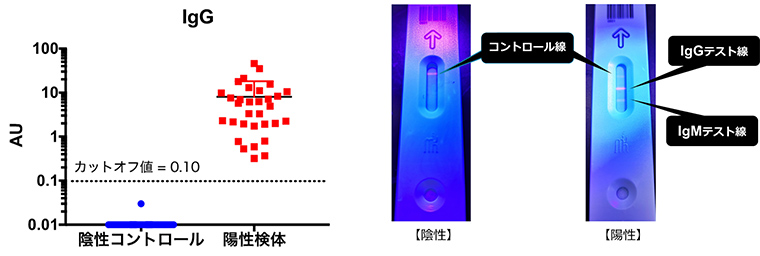
Since April, 2020, we have been collaborating through a consortium founded for COVID-19 seroepidemiological studies in Japan. SARS-CoV-2 specific antibodies were efficiently detected from COVID-19 patients using our point-of-care test. The performance characteristic of the test has been optimized through multilateral validation by way of ELISA etc. Viral neutralizability of the measured antibodies is an essential profile for developing antibody-based treatment/preventive measures.
Since June, 2020, OCU Parasitology and the Research Center for Infectious Disease Sciences(RCIDS) have signed a collaboration agreement with the Kyoto University Hospital and the Center for iPS Cell Research (CiRA). The team is exploring for novel therapeutics, establishing an optimized algorithm for COVID-19 diagnosis by combining molecular viral detection and host serology, and performing epidemiological studies.

Expected Achievements
A clinically reliable, point-of-care, serological diagnostic will be established. The neutralizability of the measured antibodies will be analyzed and its applicability for therapeutic purposes will be sought for. Moreover, fragile health-care systems in resource limited, low- or mid-income countries are sure to face increasing difficulties in medical decision making. Researchers are convinced that their point-of-care testing device will perfectly fit into such settings, where healthcare systems rely heavily on mobile medical units or local clinics. Through collaboration with their partners from the low- or mid-income countries, the group hopes to help implement this validated testing device so as to improve the preparedness of those countries for the pandemic.。
Collaborating institutions
Japan: Osaka Prefecture University, Oita University, Aichi Medical University, Keio University, St. Marianna University, Osaka Medical University, Teikyo University, Tokyo University Institute of Medicinal Science, Kyoto University Hospital and the Center for iPS Cell Research and Application, National Center Global Health and Medicine, National Institute of Infectious Diseases, VLP Therapeutics Japan
International collaborations: Institut National de la Recherche Biomédicale (Democratic Republic of the Congo), National Rosales Hospital (El Salvador)
Links
Press Release
https://www.osaka-cu.ac.jp/ja/news/2020/200427
Seroepidemiological survey lead by the Ministry of Health, Labour, and Welfare, Japan
https://www.mhlw.go.jp/content/000637285.pdf
https://www.mhlw.go.jp/content/000640287.pdf
OCU performs SARS-CoV-2 PCR examination for all applicants in order to resume face-to-face classes
https://www.osaka-cu.ac.jp/ja/news/2020/200331-3
Japan Agency for Medical Research and Development (AMED)
https://www.amed.go.jp/koubo/03/01/0301C_00059.html